The problem
Insecure manufacturing processes
Data integrity is critical in GMP-regulated environments. Regulations from the FDA, EMA, and other national bodies require the clear identification of individuals performing each action in the manufacturing process.
However, in many cases, identification relies on usernames and passwords, which create security and convenience issues. This also increases the risk of unauthorized employees using equipment without proper training.

The problem
Insecure manufacturing processes
Data integrity is critical in GMP-regulated environments. Regulations from the FDA, EMA, and other national bodies require the clear identification of individuals performing each action in the manufacturing process.
However, in many cases, identification relies on usernames and passwords, which create security and convenience issues. This also increases the risk of unauthorized employees using equipment without proper training.

Consequences
Consequences
Consequences
The solution
Biometrics in the pharmaceutical sector: Verázial•ID Pharma
The solution
Biometrics in the pharmaceutical sector: Verázial•ID Pharma
The solution
Biometrics in the pharmaceutical sector: Verázial•ID Pharma
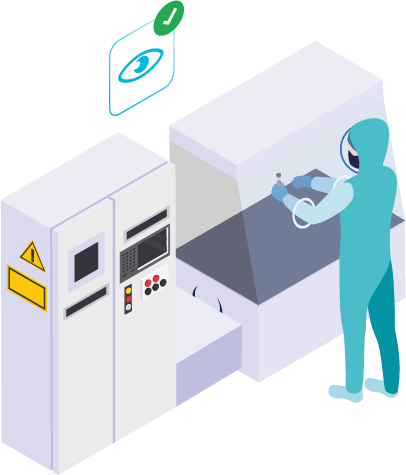
100% security in employee identification, ensuring data integrity and secure access to equipment usage.
Ease of use: The employee is authenticated by simply looking at the iris camera for a second, even while wearing gloves, masks, and/or goggles.
Efficiency improvement through faster identification processes: Employees save nearly a week per year previously spent on authentication, while the IT team saves time by no longer having to manage passwords

100% security in employee identification, ensuring data integrity and secure access to equipment usage.
Ease of use: The employee is authenticated by simply looking at the iris camera for a second, even while wearing gloves, masks, and/or goggles.
Efficiency improvement due to the speed in the process of identification. The employee saves almost a week a year of time spent on authenticatios. The IT team saves time by not having to manage passwords.

100% security in employee identification, ensuring data integrity and secure access to equipment usage.
Ease of use: The employee is authenticated by simply looking at the iris camera for a second, even while wearing gloves, masks, and/or goggles.
Efficiency improvement through faster identification processes: Employees save nearly a week per year previously spent on authentication, while the IT team saves time by no longer having to manage passwords.
Secure identification process designed for the pharmaceutical industry
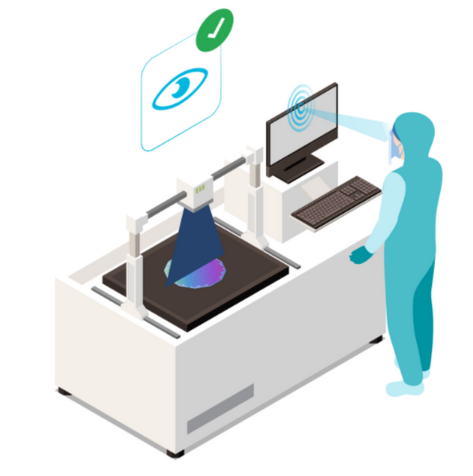
Uses the most advanced iris biometric technology for fast, error-free identification
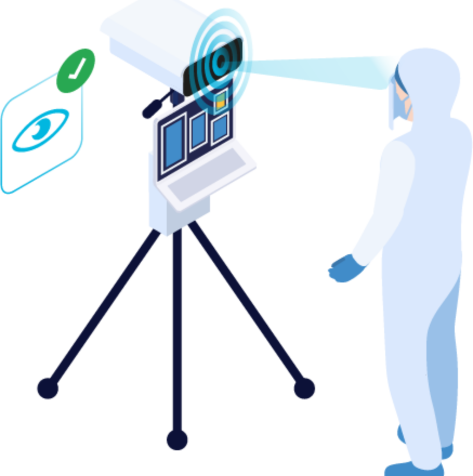
Contactless iris capture, even through protection goggles. Suitable for clean rooms.

It seamlessly integrates with all software and systems, including ERPs, MES, EBR, LIMS, and more.
Secure identification process designed for the pharmaceutical industry

Uses the most advanced iris biometric technology for fast, error-free identification.

Contactless iris capture, even through protection goggles. Suitable for clean rooms.

It seamlessly integrates with all software and systems, including ERPs, MES, EBR, LIMS, and more.
Secure identification process designed for the pharmaceutical industry

Uses the most advanced iris biometric technology for fast, error-free identification.

Contactless iris capture, even through protection goggles. Suitable for clean rooms.

It seamlessly integrates with all software and systems, including ERPs, MES, EBR, LIMS, and more.
Let´s talk
We assess how our solution can best fit your production plant’s processes.
Let´s talk
We assess how our solution can best fit your production plant’s processes.
Let´s talk
We assess how our solution can best fit your production plant’s processes.





