Employees’ identification in the pharmaceutical industry according ALCOA+
The ALCOA+ requirements defined by the FDA establish, among others, the characteristics for correct identification of employees in regulated pharmaceutical processes. The use of biometrics solves the current difficulties in meeting them.
In the pharmaceutical industry, as we addressed in a previous article , data integrity is a fundamental part of GMP (Good Manufacturing Practices) , and includes, among other issues, the obligations to be met for the secure identification of the employees who perform each task in the manufacturing processes.
Right identification according to ALCOA+
To ensure data integrity in the pharmaceutical sector, it is necessary to comply with the essential principles known as ALCOA+, as recommended by regulatory agencies such as the FDA (Food and Drug Administration, USA) or the EMA (European Medicines Agency, EU).
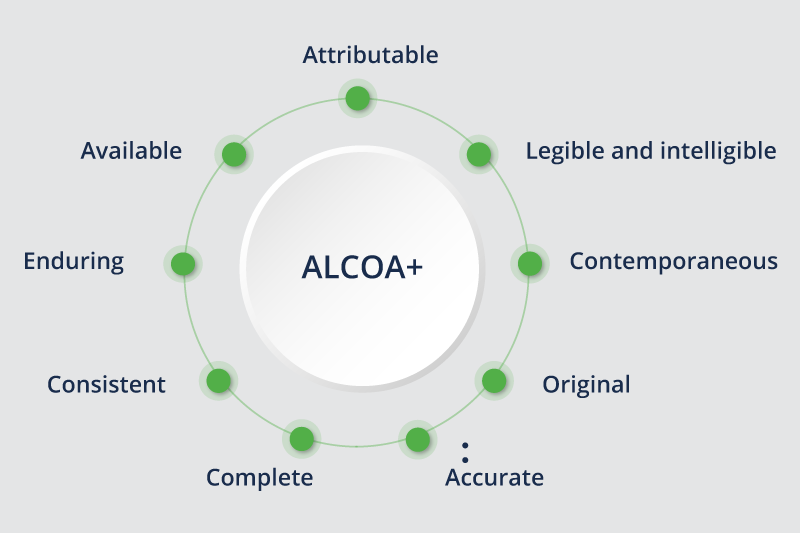
The acronym ALCOA+, defined by the FDA, refers to the attributes that data must have in order to be accurate and consistent, ensuring that it is attributable (who recorded the data and when it was recorded), readable (in the current moment and in the future), contemporaneous (recorded at the instant they are obtained) and original, among others.
Metadata is the information that describes the attributes of the data, providing context and meaning, and without which the data loses value and its integrity can’t be ensured.
The person performing a weighing, for example, is one of the metadata of the actual weighing of a certain component.
The Audit Trail is the secure electronic record that allows the reconstruction of events related to the creation, modification or deletion of electronic records.
The most relevant parameters of ALCOA+ related to the correct identification of the employees who perform the activities are:
- The ATTRIBUTABLE requirement of the ALCOA+ rule states that the person performing the activity that generates or modifies data must be identified in the record of the regulated tasks. The record of the regulated task must be attributed to the person who performed it, without any doubt.
- The LEGIBLE requirement states that the data and metadata in the records of the regulated task records must have legible and unambiguous information. The Audit Trail record in the computerized system of the regulated tasks shall contain, among others, the user’s correct identification data as part of its data and metadata.
Difficulties of current processes to comply with ALCOA+
The use of good practices according ALCOA+ to ensure data integrity in any production control system in the pharmaceutical industry involves, among other issues, being able to verify the authentication and authorization requirements of employees for each task performed.
In digitized environments where an MES (Manufacturing Execution System) or ERP (Enterprise Resource Planning) is available, authentication will be carried out by an electronic signature for each task with the introduction of login + password. This will require carrying out numerous tasks in the following areas, which represent a significant workload for the company.
- User management: the licensing to generate one user for each member of the organization.
- Control of user authentication by different access methods, such as the use of keys, swipe cards, or personal codes with passwords.
- Control of concurrent accesses that the same user can have and control of user permissions.
- Access log, preventing unauthorized access due to incorrect password, if this authentication option is used.
- Access blocking after several attempts entering a wrong password.
- Inactivity session blocking, to prevent identity theft or unauthorized access. Concurrent opening of several user sessions.
- Password management: when using electronic signature: complexity, periodic renewal, request for change due to forgetting, control of password repetition in a defined time window.

In non-digitized environments, where production control is carried out on paper, the actions mentioned will not be carried out, but it is much more difficult to comply with the premises of ALCOA+. The controls cannot be so exhaustive, everything depends much more on the human side, a lot of time is wasted working on paper and many more errors are made.
In digitized processes, an employee can sign for another simply by knowing the password. And in paper based processes, imitating the handwritten signature.
As a consequence, data integrity is at risk. We will have actions that are not clearly attributable, either due to errors in the identification or interpretation of the signature, or due to identity theft in the signature process.
And we will also have some illegible or incorrect information, and/or without an audit trail of the tasks.
Biometrics as a solution for ALCOA+ compliance and data integrity assurance
The solution that allows us to fully comply with ALCOA+, ensure data integrity, maximize employee convenience, and minimize the time spent on identification tasks and their verifications, involves the use of biometrics.
Biometrics allow us to identify ourselves with our body, thereby avoiding any possibility of error or identity theft. And since we don’t have to carry anything or remember anything, it is very convenient and no time is wasted.
In later articles on this blog we describe with more detail the problems of identification in current drug manufacturing processes, and how to solve them with the use of biometrics.
Find out how Verázial ID Pharma solves all employees’ identification problems in the pharmaceutical manufacturing processes.
Contact us for a demo and/or a customized analysis.
References
- Factory worker in blue suit [Image taken from Freepik]
Employees’ identification in the pharmaceutical industry according ALCOA+
The ALCOA+ requirements defined by the FDA establish, among others, the characteristics for correct identification of employees in regulated pharmaceutical processes. The use of biometrics solves the current difficulties in meeting them.
In the pharmaceutical industry, as we addressed in a previous article , data integrity is a fundamental part of GMP (Good Manufacturing Practices) , and includes, among other issues, the obligations to be met for the secure identification of the employees who perform each task in the manufacturing processes.
Right identification according to ALCOA+
To ensure data integrity in the pharmaceutical sector, it is necessary to comply with the essential principles known as ALCOA+, as recommended by regulatory agencies such as the FDA (Food and Drug Administration, USA) or the EMA (European Medicines Agency, EU).
The acronym ALCOA+, defined by the FDA, refers to the attributes that data must have in order to be accurate and consistent, ensuring that it is attributable (who recorded the data and when it was recorded), readable (in the current moment and in the future), contemporaneous (recorded at the instant they are obtained) and original, among others.

Metadata is the information that describes the attributes of the data, providing context and meaning, and without which the data loses value and its integrity can’t be ensured.
The person performing a weighing, for example, is one of the metadata of the actual weighing of a certain component.
The Audit Trail is the secure electronic record that allows the reconstruction of events related to the creation, modification or deletion of electronic records.
The most relevant parameters of ALCOA+ related to the correct identification of the employees who perform the activities are:
- The ATTRIBUTABLE requirement of the ALCOA+ rule states that the person performing the activity that generates or modifies data must be identified in the record of the regulated tasks. The record of the regulated task must be attributed to the person who performed it, without any doubt.
- The LEGIBLE requirement states that the data and metadata in the records of the regulated task records must have legible and unambiguous information. The Audit Trail record in the computerized system of the regulated tasks shall contain, among others, the user’s correct identification data as part of its data and metadata.
Difficulties of current processes to comply with ALCOA+
The use of good practices according ALCOA+ to ensure data integrity in any production control system in the pharmaceutical industry involves, among other issues, being able to verify the authentication and authorization requirements of employees for each task performed.
In digitized environments where an MES (Manufacturing Execution System) or ERP (Enterprise Resource Planning) is available, authentication will be carried out by an electronic signature for each task with the introduction of login + password. This will require carrying out numerous tasks in the following areas, which represent a significant workload for the company.
- User management: the licensing to generate one user for each member of the organization.
- Control of user authentication by different access methods, such as the use of keys, swipe cards, or personal codes with passwords.
- Control of concurrent accesses that the same user can have and control of user permissions.
- Access log, preventing unauthorized access due to incorrect password, if this authentication option is used.
- Access blocking after several attempts entering a wrong password.
- Inactivity session blocking, to prevent identity theft or unauthorized access. Concurrent opening of several user sessions.
- Password management: when using electronic signature: complexity, periodic renewal, request for change due to forgetting, control of password repetition in a defined time window.

In non-digitized environments, where production control is carried out on paper, the actions mentioned will not be carried out, but it is much more difficult to comply with the premises of ALCOA+. The controls cannot be so exhaustive, everything depends much more on the human side, a lot of time is wasted working on paper and many more errors are made.
In digitized processes, an employee can sign for another simply by knowing the password. And in paper based processes, imitating the handwritten signature.
As a consequence, data integrity is at risk. We will have actions that are not clearly attributable, either due to errors in the identification or interpretation of the signature, or due to identity theft in the signature process.
And we will also have some illegible or incorrect information, and/or without an audit trail of the tasks.
Biometrics as a solution for ALCOA+ compliance and data integrity assurance
The solution that allows us to fully comply with ALCOA+, ensure data integrity, maximize employee convenience, and minimize the time spent on identification tasks and their verifications, involves the use of biometrics.
Biometrics allow us to identify ourselves with our body, thereby avoiding any possibility of error or identity theft. And since we don’t have to carry anything or remember anything, it is very convenient and no time is wasted.
In later articles on this blog we describe with more detail the problems of identification in current drug manufacturing processes, and how to solve them with the use of biometrics.
Find out how Verázial ID Pharma solves all employees’ identification problems in the pharmaceutical manufacturing processes.
Contact us for a demo and/or a customized analysis.
References
- Factory worker in blue suit [Image taken from Freepik]
Employees’ identification in the pharmaceutical industry according ALCOA+
The ALCOA+ requirements defined by the FDA establish, among others, the characteristics for correct identification of employees in regulated pharmaceutical processes. The use of biometrics solves the current difficulties in meeting them.
In the pharmaceutical industry, as we addressed in a previous article , data integrity is a fundamental part of GMP (Good Manufacturing Practices) , and includes, among other issues, the obligations to be met for the secure identification of the employees who perform each task in the manufacturing processes.
Right identification according to ALCOA+
To ensure data integrity in the pharmaceutical sector, it is necessary to comply with the essential principles known as ALCOA+, as recommended by regulatory agencies such as the FDA (Food and Drug Administration, USA) or the EMA (European Medicines Agency, EU).
The acronym ALCOA+, defined by the FDA, refers to the attributes that data must have in order to be accurate and consistent, ensuring that it is attributable (who recorded the data and when it was recorded), readable (in the current moment and in the future), contemporaneous (recorded at the instant they are obtained) and original, among others.

Metadata is the information that describes the attributes of the data, providing context and meaning, and without which the data loses value and its integrity can’t be ensured.
The person performing a weighing, for example, is one of the metadata of the actual weighing of a certain component.
The Audit Trail is the secure electronic record that allows the reconstruction of events related to the creation, modification or deletion of electronic records.
The most relevant parameters of ALCOA+ related to the correct identification of the employees who perform the activities are:
- The ATTRIBUTABLE requirement of the ALCOA+ rule states that the person performing the activity that generates or modifies data must be identified in the record of the regulated tasks. The record of the regulated task must be attributed to the person who performed it, without any doubt.
- The LEGIBLE requirement states that the data and metadata in the records of the regulated task records must have legible and unambiguous information. The Audit Trail record in the computerized system of the regulated tasks shall contain, among others, the user’s correct identification data as part of its data and metadata.
Difficulties of current processes to comply with ALCOA+
The use of good practices according ALCOA+ to ensure data integrity in any production control system in the pharmaceutical industry involves, among other issues, being able to verify the authentication and authorization requirements of employees for each task performed.
In digitized environments where an MES (Manufacturing Execution System) or ERP (Enterprise Resource Planning) is available, authentication will be carried out by an electronic signature for each task with the introduction of login + password. This will require carrying out numerous tasks in the following areas, which represent a significant workload for the company.
- User management: the licensing to generate one user for each member of the organization.
- Control of user authentication by different access methods, such as the use of keys, swipe cards, or personal codes with passwords.
- Control of concurrent accesses that the same user can have and control of user permissions.
- Access log, preventing unauthorized access due to incorrect password, if this authentication option is used.
- Access blocking after several attempts entering a wrong password.
- Inactivity session blocking, to prevent identity theft or unauthorized access. Concurrent opening of several user sessions.
- Password management: when using electronic signature: complexity, periodic renewal, request for change due to forgetting, control of password repetition in a defined time window.

In non-digitized environments, where production control is carried out on paper, the actions mentioned will not be carried out, but it is much more difficult to comply with the premises of ALCOA+. The controls cannot be so exhaustive, everything depends much more on the human side, a lot of time is wasted working on paper and many more errors are made.
In digitized processes, an employee can sign for another simply by knowing the password. And in paper based processes, imitating the handwritten signature.
As a consequence, data integrity is at risk. We will have actions that are not clearly attributable, either due to errors in the identification or interpretation of the signature, or due to identity theft in the signature process.
And we will also have some illegible or incorrect information, and/or without an audit trail of the tasks.
Biometrics as a solution for ALCOA+ compliance and data integrity assurance
The solution that allows us to fully comply with ALCOA+, ensure data integrity, maximize employee convenience, and minimize the time spent on identification tasks and their verifications, involves the use of biometrics.
Biometrics allow us to identify ourselves with our body, thereby avoiding any possibility of error or identity theft. And since we don’t have to carry anything or remember anything, it is very convenient and no time is wasted.
In later articles on this blog we describe with more detail the problems of identification in current drug manufacturing processes, and how to solve them with the use of biometrics.
Find out how Verázial ID Pharma solves all employees’ identification problems in the pharmaceutical manufacturing processes.
Contact us for a demo and/or a customized analysis.
References
- Factory worker in blue suit [Image taken from Freepik]
You May Also Like
You May Also Like





