FDA inspections in the pharmaceutical industry
The FDA and EMA share the goal of ensuring compliance with GMP and other applicable regulations in pharmaceutical manufacturing. These entities carry out exhaustive inspections of companies to verify their compliance with established standards. Is your company ready to face the rigorous inspection processes?
Due to the very essence of its operations, the pharmaceutical industry is obligated to comply with strict quality regulations. These regulations not only affect drug production, but also extend to all elements of the value chain. The regulations are based on very similar standards. The main regulatory bodies worldwide are:
As an example of national regulations, for example the Spanish, we have the AEMPS, the Spanish Agency of Medicines and Health Products, which in this case strictly follows the EMA regulations as a member country of the EU.
These regulatory agencies have the task of supervising the manufacture, marketing and distribution of various products such as drugs, medical devices, biological products, veterinary products and cosmetics. The fundamental objective is to ensure compliance with GMP (Good Manufacturing Practices) and other applicable regulations in the manufacture of pharmaceutical products.
Regardless of their geographic location, many pharmaceutical companies market their products in the United States or intend to do so, which entails the obligation to undergo an inspection process of their facilities and processes carried out by the FDA. These inspections are conducted directly at the companies’ facilities and manufacturing areas. We will now turn our attention to the FDA, both because of this circumstance and because it is the main reference for the rest of the agencies worldwide.
The FDA can conduct 4 different types of inspections:
- Pre-Approval Inspections (PAI): These inspections are critical reviews conducted by the FDA when a company applies for approval to sell a new product or install a new manufacturing facility. These inspections focus on verifying the information submitted in the application and ensuring that the facility can effectively produce the product. The outcome of an IAP is a recommendation by the inspectors for or against approval by the FDA. Applications include NDA (New Drug Application) for new drugs, ANDA (Abbreviated New Drug Application) for generic drugs, BLA (Biologics License Application) for biologics and PMA (Pre-market Approval) for high-risk medical devices.
- Surveillance inspections: These routine reviews, in compliance with GMP for drugs or the quality system, are mandatory by law every 2-3 years for product manufacturers. It is crucial to note that currently these reviews are conducted on a surprise, unannounced basis, which highlights the importance of continuous preparation of companies to ensure successful approval in these “surprise” evaluations.
- Follow-up compliance inspections: These reviews evaluate actions taken by a company or manufacturer in response to a previous inspection that generated significant observations or a Warning Letter. The purpose of these follow-up inspections is to verify the proper correction of previous observations, to document if they have not yet been corrected, or to support possible future regulatory actions.
- “For Cause” Inspections: These reviews are conducted to investigate a specific problem that has been reported to FDA. The source of the report may come from the manufacturer, as a result of a recall, consumer or user complaints, or adverse effects reported by medical personnel. A “for cause” inspection will focus on the particular problem, but may be extended to address unrelated aspects of the company’s operations.
Currently, there is a Mutual Recognition Agreement (MRA) between the FDA and regulatory authorities of the European Union, Switzerland, and the United Kingdom for inspections of Good Manufacturing Practices (GMP). This agreement allows the recognition of drug inspections conducted by these authorities, provided that the FDA determines they meet the requirements set by the United States. The MRA enhances efficiency in both U.S. and foreign regulatory systems by avoiding duplicate inspections.
This cooperation involves sharing data on possible side effects, supervising clinical trials and the general results obtained when carrying out inspections at manufacturers’ facilities where they evaluate and verify compliance with regulations, all with the aim of effectively regulating medicines, maintaining high standards of quality and safety for the benefit of patients.
It is important to note that the existence of this agreement does not imply that FDA will no longer conduct inspections in the European Union. Both FDA and the EMA in the EU have the right to conduct inspections at any time and in any country. However, FDA surveillance inspections in the EU are expected to be less frequent. Following a positive capability assessment, the FDA will recognize the EU authorities (EMA) as competent and will therefore accept their inspections of drug manufacturing facilities.
Similarly, the FDA specifies that both it and the European Union make decisions independently after an inspection, based on the findings of each. Although enforcement actions may have similar consequences, the two entities have different legal systems and enforcement methods, which may lead to different actions being taken in response to a similar situation.
What is the FDA inspection process like?
To ensure a successful inspection, it is crucial to consider five elements that will affect the final outcome of the review. Companies should recognize the importance of these factors and prepare adequately to effectively address inspections. The following are recommendations on key aspects to consider in order to successfully prepare for and successfully pass the inspection process:
- Quality systems: Quality systems must first be brought into compliance with FDA regulations and a work plan (gap assessment) will be developed to address and correct any observations that arise during the FDA inspection.
- Facilities: As immediate actions, initial internal audits should be conducted and facility visits should be planned on a weekly basis. Any damage identified in the facilities should be addressed with appropriate repairs. In addition, constant checking should be carried out to ensure that equipment is functioning properly. In addition, it is essential to carry out a review of the external condition of the facilities and evaluate access control to it.
- Personnel: Involving the entire team in the inspection is key. Defining roles, briefing and training personnel are essential steps. Identifying experts in the inspected area and evaluating their skills is fundamental. Performing simulations of the process, from reception to dismissal, allows for testing communication systems and document presentation.
- Inspector: When you know the person who will carry out the inspection, it is essential to know his profile, including his experience and level of knowledge in the evaluated areas. This information is used to adequately inform the person interacting with the inspector, thus contributing to create an environment of empathy and mutual understanding between all parties involved.
- Inspection process: The process is based on the following flow.
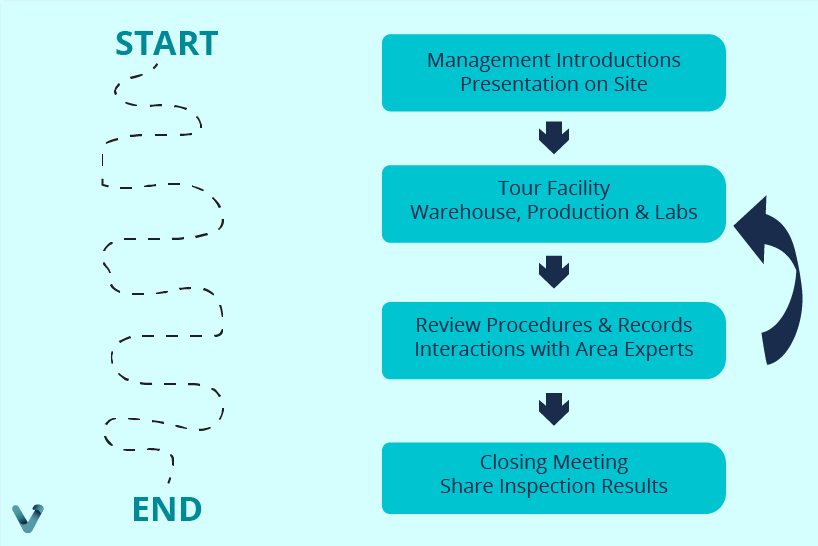
Preparing for a successful inspection involves understanding the relevant terminology and the “Compliance Program,” which ensures compliance with specific regulations. Proper plant presentation, creating “Standard Operating Procedures” (SOPs) and simulating inspections are essential steps. During the inspection, following a planned script, anticipating documentation and monitoring the interaction with the inspector are crucial practices.
Following inspections, companies that fail to comply with FDA standards, especially GMP regulations, can face significant legal and regulatory repercussions such as the issuance of Warning Letters, product recalls, suspension of operations, import or export restrictions, legal action and lawsuits, as well as reputational damage. In a future article, each of these legal consequences will be detailed in detail.
As we have mentioned in previous articles, GMP environments regulate several factors, including the critical obligation to safeguard the integrity of the data collected, which includes the secure identification of employees involved in each phase of the manufacturing processes.
Current methods of employee identification and process control in the pharmaceutical industry present challenges in terms of security, which puts data integrity and traceability at risk, thus affecting compliance with established regulations. To address the dysfunctions and challenges the optimal solution involves the implementation of biometrics for secure and convenient identification of employees. Biometrics allows identification through body characteristics, eliminating any possibility of error or impersonation. We invite you to read our article Biometric electronic signature in pharmaceutical manufacturing processes for more information.
Find out how Verázial ID Pharma helps you in the inspection process of the FDA and other regulatory agencies, ensuring data integrity in the identification process.
Contact us for a demonstration and/or personalized study.
FDA inspections in the pharmaceutical industry
The FDA and EMA share the goal of ensuring compliance with GMP and other applicable regulations in pharmaceutical manufacturing. These entities carry out exhaustive inspections of companies to verify their compliance with established standards. Is your company ready to face the rigorous inspection processes?
Due to the very essence of its operations, the pharmaceutical industry is obligated to comply with strict quality regulations. These regulations not only affect drug production, but also extend to all elements of the value chain. The regulations are based on very similar standards. The main regulatory bodies worldwide are:
As an example of national regulations, for example the Spanish, we have the AEMPS, the Spanish Agency of Medicines and Health Products, which in this case strictly follows the EMA regulations as a member country of the EU.
These regulatory agencies have the task of supervising the manufacture, marketing and distribution of various products such as drugs, medical devices, biological products, veterinary products and cosmetics. The fundamental objective is to ensure compliance with GMP (Good Manufacturing Practices) and other applicable regulations in the manufacture of pharmaceutical products.
Regardless of their geographic location, many pharmaceutical companies market their products in the United States or intend to do so, which entails the obligation to undergo an inspection process of their facilities and processes carried out by the FDA. These inspections are conducted directly at the companies’ facilities and manufacturing areas. We will now turn our attention to the FDA, both because of this circumstance and because it is the main reference for the rest of the agencies worldwide.
The FDA can conduct 4 different types of inspections:
- Pre-Approval Inspections (PAI): These inspections are critical reviews conducted by the FDA when a company applies for approval to sell a new product or install a new manufacturing facility. These inspections focus on verifying the information submitted in the application and ensuring that the facility can effectively produce the product. The outcome of an IAP is a recommendation by the inspectors for or against approval by the FDA. Applications include NDA (New Drug Application) for new drugs, ANDA (Abbreviated New Drug Application) for generic drugs, BLA (Biologics License Application) for biologics and PMA (Pre-market Approval) for high-risk medical devices.
- Surveillance inspections: These routine reviews, in compliance with GMP for drugs or the quality system, are mandatory by law every 2-3 years for product manufacturers. It is crucial to note that currently these reviews are conducted on a surprise, unannounced basis, which highlights the importance of continuous preparation of companies to ensure successful approval in these “surprise” evaluations.
- Follow-up compliance inspections: These reviews evaluate actions taken by a company or manufacturer in response to a previous inspection that generated significant observations or a Warning Letter. The purpose of these follow-up inspections is to verify the proper correction of previous observations, to document if they have not yet been corrected, or to support possible future regulatory actions.
- “For Cause” Inspections: These reviews are conducted to investigate a specific problem that has been reported to FDA. The source of the report may come from the manufacturer, as a result of a recall, consumer or user complaints, or adverse effects reported by medical personnel. A “for cause” inspection will focus on the particular problem, but may be extended to address unrelated aspects of the company’s operations.
Currently, there is a Mutual Recognition Agreement (MRA) between the FDA and regulatory authorities of the European Union, Switzerland, and the United Kingdom for inspections of Good Manufacturing Practices (GMP). This agreement allows the recognition of drug inspections conducted by these authorities, provided that the FDA determines they meet the requirements set by the United States. The MRA enhances efficiency in both U.S. and foreign regulatory systems by avoiding duplicate inspections.
This cooperation involves sharing data on possible side effects, supervising clinical trials and the general results obtained when carrying out inspections at manufacturers’ facilities where they evaluate and verify compliance with regulations, all with the aim of effectively regulating medicines, maintaining high standards of quality and safety for the benefit of patients.
It is important to note that the existence of this agreement does not imply that FDA will no longer conduct inspections in the European Union. Both FDA and the EMA in the EU have the right to conduct inspections at any time and in any country. However, FDA surveillance inspections in the EU are expected to be less frequent. Following a positive capability assessment, the FDA will recognize the EU authorities (EMA) as competent and will therefore accept their inspections of drug manufacturing facilities.
Similarly, the FDA specifies that both it and the European Union make decisions independently after an inspection, based on the findings of each. Although enforcement actions may have similar consequences, the two entities have different legal systems and enforcement methods, which may lead to different actions being taken in response to a similar situation.
What is the FDA inspection process like?
To ensure a successful inspection, it is crucial to consider five elements that will affect the final outcome of the review. Companies should recognize the importance of these factors and prepare adequately to effectively address inspections. The following are recommendations on key aspects to consider in order to successfully prepare for and successfully pass the inspection process:
- Quality systems: Quality systems must first be brought into compliance with FDA regulations and a work plan (gap assessment) will be developed to address and correct any observations that arise during the FDA inspection.
- Facilities: As immediate actions, initial internal audits should be conducted and facility visits should be planned on a weekly basis. Any damage identified in the facilities should be addressed with appropriate repairs. In addition, constant checking should be carried out to ensure that equipment is functioning properly. In addition, it is essential to carry out a review of the external condition of the facilities and evaluate access control to it.
- Personnel: Involving the entire team in the inspection is key. Defining roles, briefing and training personnel are essential steps. Identifying experts in the inspected area and evaluating their skills is fundamental. Performing simulations of the process, from reception to dismissal, allows for testing communication systems and document presentation.
- Inspector: When you know the person who will carry out the inspection, it is essential to know his profile, including his experience and level of knowledge in the evaluated areas. This information is used to adequately inform the person interacting with the inspector, thus contributing to create an environment of empathy and mutual understanding between all parties involved.
- Inspection process: The process is based on the following flow.

Preparing for a successful inspection involves understanding the relevant terminology and the “Compliance Program,” which ensures compliance with specific regulations. Proper plant presentation, creating “Standard Operating Procedures” (SOPs) and simulating inspections are essential steps. During the inspection, following a planned script, anticipating documentation and monitoring the interaction with the inspector are crucial practices.
Following inspections, companies that fail to comply with FDA standards, especially GMP regulations, can face significant legal and regulatory repercussions such as the issuance of Warning Letters, product recalls, suspension of operations, import or export restrictions, legal action and lawsuits, as well as reputational damage. In a future article, each of these legal consequences will be detailed in detail.
As we have mentioned in previous articles, GMP environments regulate several factors, including the critical obligation to safeguard the integrity of the data collected, which includes the secure identification of employees involved in each phase of the manufacturing processes.
Current methods of employee identification and process control in the pharmaceutical industry present challenges in terms of security, which puts data integrity and traceability at risk, thus affecting compliance with established regulations. To address the dysfunctions and challenges the optimal solution involves the implementation of biometrics for secure and convenient identification of employees. Biometrics allows identification through body characteristics, eliminating any possibility of error or impersonation. We invite you to read our article Biometric electronic signature in pharmaceutical manufacturing processes for more information.
Find out how Verázial ID Pharma helps you in the inspection process of the FDA and other regulatory agencies, ensuring data integrity in the identification process.
Contact us for a demonstration and/or personalized study.
FDA inspections in the pharmaceutical industry
The FDA and EMA share the goal of ensuring compliance with GMP and other applicable regulations in pharmaceutical manufacturing. These entities carry out exhaustive inspections of companies to verify their compliance with established standards. Is your company ready to face the rigorous inspection processes?
Due to the very essence of its operations, the pharmaceutical industry is obligated to comply with strict quality regulations. These regulations not only affect drug production, but also extend to all elements of the value chain. The regulations are based on very similar standards. The main regulatory bodies worldwide are:
As an example of national regulations, for example the Spanish, we have the AEMPS, the Spanish Agency of Medicines and Health Products, which in this case strictly follows the EMA regulations as a member country of the EU.
These regulatory agencies have the task of supervising the manufacture, marketing and distribution of various products such as drugs, medical devices, biological products, veterinary products and cosmetics. The fundamental objective is to ensure compliance with GMP (Good Manufacturing Practices) and other applicable regulations in the manufacture of pharmaceutical products.
Regardless of their geographic location, many pharmaceutical companies market their products in the United States or intend to do so, which entails the obligation to undergo an inspection process of their facilities and processes carried out by the FDA. These inspections are conducted directly at the companies’ facilities and manufacturing areas. We will now turn our attention to the FDA, both because of this circumstance and because it is the main reference for the rest of the agencies worldwide.
The FDA can conduct 4 different types of inspections:
- Pre-Approval Inspections (PAI): These inspections are critical reviews conducted by the FDA when a company applies for approval to sell a new product or install a new manufacturing facility. These inspections focus on verifying the information submitted in the application and ensuring that the facility can effectively produce the product. The outcome of an IAP is a recommendation by the inspectors for or against approval by the FDA. Applications include NDA (New Drug Application) for new drugs, ANDA (Abbreviated New Drug Application) for generic drugs, BLA (Biologics License Application) for biologics and PMA (Pre-market Approval) for high-risk medical devices.
- Surveillance inspections: These routine reviews, in compliance with GMP for drugs or the quality system, are mandatory by law every 2-3 years for product manufacturers. It is crucial to note that currently these reviews are conducted on a surprise, unannounced basis, which highlights the importance of continuous preparation of companies to ensure successful approval in these “surprise” evaluations.
- Follow-up compliance inspections: These reviews evaluate actions taken by a company or manufacturer in response to a previous inspection that generated significant observations or a Warning Letter. The purpose of these follow-up inspections is to verify the proper correction of previous observations, to document if they have not yet been corrected, or to support possible future regulatory actions.
- “For Cause” Inspections: These reviews are conducted to investigate a specific problem that has been reported to FDA. The source of the report may come from the manufacturer, as a result of a recall, consumer or user complaints, or adverse effects reported by medical personnel. A “for cause” inspection will focus on the particular problem, but may be extended to address unrelated aspects of the company’s operations.
Currently, there is a Mutual Recognition Agreement (MRA) between the FDA and regulatory authorities of the European Union, Switzerland, and the United Kingdom for inspections of Good Manufacturing Practices (GMP). This agreement allows the recognition of drug inspections conducted by these authorities, provided that the FDA determines they meet the requirements set by the United States. The MRA enhances efficiency in both U.S. and foreign regulatory systems by avoiding duplicate inspections.
This cooperation involves sharing data on possible side effects, supervising clinical trials and the general results obtained when carrying out inspections at manufacturers’ facilities where they evaluate and verify compliance with regulations, all with the aim of effectively regulating medicines, maintaining high standards of quality and safety for the benefit of patients.
It is important to note that the existence of this agreement does not imply that FDA will no longer conduct inspections in the European Union. Both FDA and the EMA in the EU have the right to conduct inspections at any time and in any country. However, FDA surveillance inspections in the EU are expected to be less frequent. Following a positive capability assessment, the FDA will recognize the EU authorities (EMA) as competent and will therefore accept their inspections of drug manufacturing facilities.
Similarly, the FDA specifies that both it and the European Union make decisions independently after an inspection, based on the findings of each. Although enforcement actions may have similar consequences, the two entities have different legal systems and enforcement methods, which may lead to different actions being taken in response to a similar situation.
What is the FDA inspection process like?
To ensure a successful inspection, it is crucial to consider five elements that will affect the final outcome of the review. Companies should recognize the importance of these factors and prepare adequately to effectively address inspections. The following are recommendations on key aspects to consider in order to successfully prepare for and successfully pass the inspection process:
- Quality systems: Quality systems must first be brought into compliance with FDA regulations and a work plan (gap assessment) will be developed to address and correct any observations that arise during the FDA inspection.
- Facilities: As immediate actions, initial internal audits should be conducted and facility visits should be planned on a weekly basis. Any damage identified in the facilities should be addressed with appropriate repairs. In addition, constant checking should be carried out to ensure that equipment is functioning properly. In addition, it is essential to carry out a review of the external condition of the facilities and evaluate access control to it.
- Personnel: Involving the entire team in the inspection is key. Defining roles, briefing and training personnel are essential steps. Identifying experts in the inspected area and evaluating their skills is fundamental. Performing simulations of the process, from reception to dismissal, allows for testing communication systems and document presentation.
- Inspector: When you know the person who will carry out the inspection, it is essential to know his profile, including his experience and level of knowledge in the evaluated areas. This information is used to adequately inform the person interacting with the inspector, thus contributing to create an environment of empathy and mutual understanding between all parties involved.
- Inspection process: The process is based on the following flow.

Preparing for a successful inspection involves understanding the relevant terminology and the “Compliance Program,” which ensures compliance with specific regulations. Proper plant presentation, creating “Standard Operating Procedures” (SOPs) and simulating inspections are essential steps. During the inspection, following a planned script, anticipating documentation and monitoring the interaction with the inspector are crucial practices.
Following inspections, companies that fail to comply with FDA standards, especially GMP regulations, can face significant legal and regulatory repercussions such as the issuance of Warning Letters, product recalls, suspension of operations, import or export restrictions, legal action and lawsuits, as well as reputational damage. In a future article, each of these legal consequences will be detailed in detail.
As we have mentioned in previous articles, GMP environments regulate several factors, including the critical obligation to safeguard the integrity of the data collected, which includes the secure identification of employees involved in each phase of the manufacturing processes.
Current methods of employee identification and process control in the pharmaceutical industry present challenges in terms of security, which puts data integrity and traceability at risk, thus affecting compliance with established regulations. To address the dysfunctions and challenges the optimal solution involves the implementation of biometrics for secure and convenient identification of employees. Biometrics allows identification through body characteristics, eliminating any possibility of error or impersonation. We invite you to read our article Biometric electronic signature in pharmaceutical manufacturing processes for more information.
Find out how Verázial ID Pharma helps you in the inspection process of the FDA and other regulatory agencies, ensuring data integrity in the identification process.
Contact us for a demonstration and/or personalized study.
You May Also Like
You May Also Like





