Audit Trail to ensure data integrity in the pharmaceutical manufacturing process
The biometric electronic signature within the audit trail action log ensures the integrity of records in GMP regulated environments, providing detailed and reliable tracking of recorded activities.
As we have evidenced in previous articles, ensuring data integrity is a mandatory requirement in GMP (Good Manufacturing Practices) regulated environments such as the pharmaceutical industry. This involves ensuring the accurate identification of the employees who carry out each step in the manufacturing processes, among other responsibilities.
To ensure data integrity in the pharmaceutical industry, it is essential to follow the essential principles known as ALCOA+, as recommended by regulatory bodies such as the FDA (U.S. Food and Drug Administration) or the EMA (European Medicines Agency of the EU).
The acronym ALCOA+, created by the FDA, refers to the criteria that data must meet to be accurate and reliable, ensuring that it is Attributable (identifying who recorded the data and when), Legible (both now and in the future), Contemporaneous (recorded at the time it is collected) and Original, among other aspects. More information can be found in our article Employees’ identification in the pharmaceutical industry according ALCOA+.
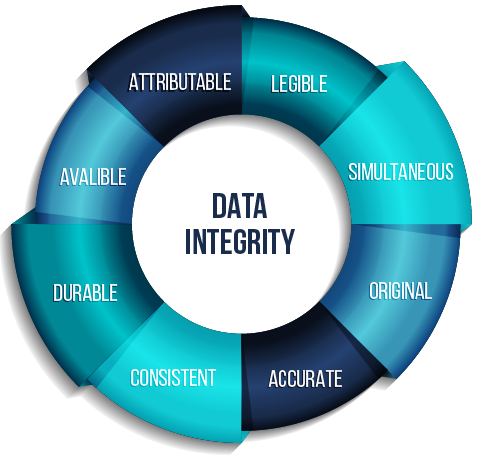
For the case that concerns us in the following article, we will place special emphasis on the requirements of attributability and legibility of the recorded data, since the ALCOA+ rule indicates that the record of the activity must be unequivocally attributed to the person who carried it out, with no room for doubt, and that it is essential that the data and metadata in the records of regulated activities be clear and easy to understand. In addition, it indicates that computerized record keeping systems, such as an Audit Trail, should include detailed information on the responsible user, along with other relevant data.
What is an Audit Trail?
Audit trails are secure electronic records that allow the reconstruction of events associated with the creation, modification or deletion of electronic records.
These records provide supporting documentation and a history to validate operational and safety actions, or to detect and counteract deviations. In other words, they serve as evidence of compliance and operational integrity. During IT system validation, the audit trail becomes an essential tool for preserving information and system integrity.
Regulatory requirements for the audit trail of a computerized system
- Identification of the origin of the data: It is necessary that the registry of regulated activities clearly identify the person, system or equipment that generates the data. To this end, system administrators are required to adequately code users and associated equipment, ensuring unique identification.
- Identification of the activity carried out: It is also crucial that the record of regulated activities identifies the person or system that carries out the activity, either by generating or modifying data.
- Complete data and metadata registry: It is essential that all relevant data and metadata be included in the registry of regulated activities.
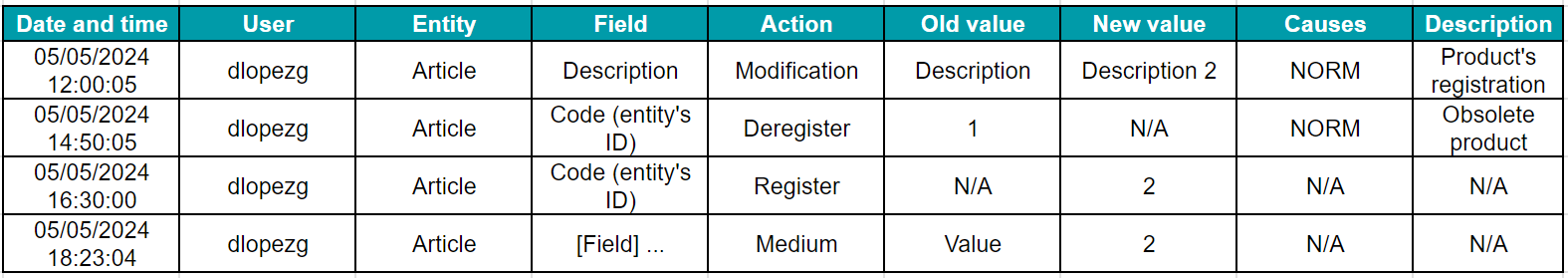
- Data and metadata associated with the audit trail: This includes the date and time of the activity, the responsible user, the affected entity, the new and old value (in case of modifications), and the reason for the change.
- Availability of original data and modifications: It must be ensured that both the original data and any subsequent modifications and results generated by the system are available for review and follow-up
Audit trail system features
The essential features of the audit trail system are designed to ensure the integrity of electronic records. In accordance with regulations and best practice standards, the audit trail system is required to meet the following requirements:
- Availability: Audit trails should always be available in a format that allows them to be reviewed by regulatory bodies.
- Automation: Entries in the audit trail should be captured automatically by the computer system, without manual intervention by users.
- Custody: The audit trail must be retained for the time required to maintain the integrity of the corresponding electronic records.
- Traceability: Each entry in the audit trail should be attributable to the person responsible for entering the data, and updates should not obscure previous values. In addition, the reason for any changes should be recorded, where required by regulation.
- Contemporaneity: Each entry in the audit trail must have a precise and non-modifiable time stamp, using a controlled clock system.
- Security: Audit trail data must be securely stored and inaccessible for editing by any user.
Example of a computerized audit trail system:
How to ensure the integrity of electronic records?
The optimal solution is the implementation of a biometric electronic signature for the identification and recording of actions within the audit trail, thus ensuring the integrity of records within GMP regulated environments.
This type of technology not only provides convenience to users by eliminating the need for extra devices or remembering passwords for their electronic signature, but also ensures traceability of actions and data integrity, fully complying with the regulatory requirements of the pharmaceutical industry.
Biometric technology leverages the unique characteristics of our body to achieve accurate and convenient identification for the employee. Examples of these features include fingerprints, face, voice and irises.
We invite you to read previous articles on our blog where we have addressed more on this topic:
Find out how Verázial ID Pharma can help you ensure data integrity in pharmaceutical manufacturing processes.
Contact us for a demonstration and/or personalized study.
References
- Unrecognized farm worker with a tablet. [Freepik]
Audit Trail to ensure data integrity in the pharmaceutical manufacturing process
The biometric electronic signature within the audit trail action log ensures the integrity of records in GMP regulated environments, providing detailed and reliable tracking of recorded activities.
As we have evidenced in previous articles, ensuring data integrity is a mandatory requirement in GMP (Good Manufacturing Practices) regulated environments such as the pharmaceutical industry. This involves ensuring the accurate identification of the employees who carry out each step in the manufacturing processes, among other responsibilities.
To ensure data integrity in the pharmaceutical industry, it is essential to follow the essential principles known as ALCOA+, as recommended by regulatory bodies such as the FDA (U.S. Food and Drug Administration) or the EMA (European Medicines Agency of the EU).
The acronym ALCOA+, created by the FDA, refers to the criteria that data must meet to be accurate and reliable, ensuring that it is Attributable (identifying who recorded the data and when), Legible (both now and in the future), Contemporaneous (recorded at the time it is collected) and Original, among other aspects. More information can be found in our article Employees’ identification in the pharmaceutical industry according ALCOA+.

For the case that concerns us in the following article, we will place special emphasis on the requirements of attributability and legibility of the recorded data, since the ALCOA+ rule indicates that the record of the activity must be unequivocally attributed to the person who carried it out, with no room for doubt, and that it is essential that the data and metadata in the records of regulated activities be clear and easy to understand. In addition, it indicates that computerized record keeping systems, such as an Audit Trail, should include detailed information on the responsible user, along with other relevant data.
What is an Audit Trail?
Audit trails are secure electronic records that allow the reconstruction of events associated with the creation, modification or deletion of electronic records.
These records provide supporting documentation and a history to validate operational and safety actions, or to detect and counteract deviations. In other words, they serve as evidence of compliance and operational integrity. During IT system validation, the audit trail becomes an essential tool for preserving information and system integrity.
Regulatory requirements for the audit trail of a computerized system
- Identification of the origin of the data: It is necessary that the registry of regulated activities clearly identify the person, system or equipment that generates the data. To this end, system administrators are required to adequately code users and associated equipment, ensuring unique identification.
- Identification of the activity carried out: It is also crucial that the record of regulated activities identifies the person or system that carries out the activity, either by generating or modifying data.
- Complete data and metadata registry: It is essential that all relevant data and metadata be included in the registry of regulated activities.
- Data and metadata associated with the audit trail: This includes the date and time of the activity, the responsible user, the affected entity, the new and old value (in case of modifications), and the reason for the change.
- Availability of original data and modifications: It must be ensured that both the original data and any subsequent modifications and results generated by the system are available for review and follow-up
Audit trail system features
The essential features of the audit trail system are designed to ensure the integrity of electronic records. In accordance with regulations and best practice standards, the audit trail system is required to meet the following requirements:
- Availability: Audit trails should always be available in a format that allows them to be reviewed by regulatory bodies.
- Automation: Entries in the audit trail should be captured automatically by the computer system, without manual intervention by users.
- Custody: The audit trail must be retained for the time required to maintain the integrity of the corresponding electronic records.
- Traceability: Each entry in the audit trail should be attributable to the person responsible for entering the data, and updates should not obscure previous values. In addition, the reason for any changes should be recorded, where required by regulation.
- Contemporaneity: Each entry in the audit trail must have a precise and non-modifiable time stamp, using a controlled clock system.
- Security: Audit trail data must be securely stored and inaccessible for editing by any user.
Example of a computerized audit trail system:

How to ensure the integrity of electronic records?
The optimal solution is the implementation of a biometric electronic signature for the identification and recording of actions within the audit trail, thus ensuring the integrity of records within GMP regulated environments.
This type of technology not only provides convenience to users by eliminating the need for extra devices or remembering passwords for their electronic signature, but also ensures traceability of actions and data integrity, fully complying with the regulatory requirements of the pharmaceutical industry.
Biometric technology leverages the unique characteristics of our body to achieve accurate and convenient identification for the employee. Examples of these features include fingerprints, face, voice and irises.
We invite you to read previous articles on our blog where we have addressed more on this topic:
Find out how Verázial ID Pharma can help you ensure data integrity in pharmaceutical manufacturing processes.
Contact us for a demonstration and/or personalized study.
References
- Unrecognized farm worker with a tablet. [Freepik]
Audit Trail to ensure data integrity in the pharmaceutical manufacturing process
The biometric electronic signature within the audit trail action log ensures the integrity of records in GMP regulated environments, providing detailed and reliable tracking of recorded activities.
As we have evidenced in previous articles, ensuring data integrity is a mandatory requirement in GMP (Good Manufacturing Practices) regulated environments such as the pharmaceutical industry. This involves ensuring the accurate identification of the employees who carry out each step in the manufacturing processes, among other responsibilities.
To ensure data integrity in the pharmaceutical industry, it is essential to follow the essential principles known as ALCOA+, as recommended by regulatory bodies such as the FDA (U.S. Food and Drug Administration) or the EMA (European Medicines Agency of the EU).
The acronym ALCOA+, created by the FDA, refers to the criteria that data must meet to be accurate and reliable, ensuring that it is Attributable (identifying who recorded the data and when), Legible (both now and in the future), Contemporaneous (recorded at the time it is collected) and Original, among other aspects. More information can be found in our article Employees’ identification in the pharmaceutical industry according ALCOA+.

For the case that concerns us in the following article, we will place special emphasis on the requirements of attributability and legibility of the recorded data, since the ALCOA+ rule indicates that the record of the activity must be unequivocally attributed to the person who carried it out, with no room for doubt, and that it is essential that the data and metadata in the records of regulated activities be clear and easy to understand. In addition, it indicates that computerized record keeping systems, such as an Audit Trail, should include detailed information on the responsible user, along with other relevant data.
What is an Audit Trail?
Audit trails are secure electronic records that allow the reconstruction of events associated with the creation, modification or deletion of electronic records.
These records provide supporting documentation and a history to validate operational and safety actions, or to detect and counteract deviations. In other words, they serve as evidence of compliance and operational integrity. During IT system validation, the audit trail becomes an essential tool for preserving information and system integrity.
Regulatory requirements for the audit trail of a computerized system
- Identification of the origin of the data: It is necessary that the registry of regulated activities clearly identify the person, system or equipment that generates the data. To this end, system administrators are required to adequately code users and associated equipment, ensuring unique identification.
- Identification of the activity carried out: It is also crucial that the record of regulated activities identifies the person or system that carries out the activity, either by generating or modifying data.
- Complete data and metadata registry: It is essential that all relevant data and metadata be included in the registry of regulated activities.
- Data and metadata associated with the audit trail: This includes the date and time of the activity, the responsible user, the affected entity, the new and old value (in case of modifications), and the reason for the change.
- Availability of original data and modifications: It must be ensured that both the original data and any subsequent modifications and results generated by the system are available for review and follow-up
Audit trail system features
The essential features of the audit trail system are designed to ensure the integrity of electronic records. In accordance with regulations and best practice standards, the audit trail system is required to meet the following requirements:
- Availability: Audit trails should always be available in a format that allows them to be reviewed by regulatory bodies.
- Automation: Entries in the audit trail should be captured automatically by the computer system, without manual intervention by users.
- Custody: The audit trail must be retained for the time required to maintain the integrity of the corresponding electronic records.
- Traceability: Each entry in the audit trail should be attributable to the person responsible for entering the data, and updates should not obscure previous values. In addition, the reason for any changes should be recorded, where required by regulation.
- Contemporaneity: Each entry in the audit trail must have a precise and non-modifiable time stamp, using a controlled clock system.
- Security: Audit trail data must be securely stored and inaccessible for editing by any user.
Example of a computerized audit trail system:

How to ensure the integrity of electronic records?
The optimal solution is the implementation of a biometric electronic signature for the identification and recording of actions within the audit trail, thus ensuring the integrity of records within GMP regulated environments.
This type of technology not only provides convenience to users by eliminating the need for extra devices or remembering passwords for their electronic signature, but also ensures traceability of actions and data integrity, fully complying with the regulatory requirements of the pharmaceutical industry.
Biometric technology leverages the unique characteristics of our body to achieve accurate and convenient identification for the employee. Examples of these features include fingerprints, face, voice and irises.
We invite you to read previous articles on our blog where we have addressed more on this topic:
Find out how Verázial ID Pharma can help you ensure data integrity in pharmaceutical manufacturing processes.
Contact us for a demonstration and/or personalized study.
References
- Unrecognized farm worker with a tablet. [Freepik]
You May Also Like
You May Also Like





